External committee opportunities

A number of UK committees appoint toxicologists to provide independent and expert advice either as a regular position or for ad hoc support. If you’re interested in furthering your involvement, you can find more details on each committee below. We also advertise these opportunities when available through email and via the website’s news page.
This is not intended to be an exhaustive list. If you are aware of a further committee of interest to BTS members, or you would like your own committee to be listed, please send relevant details to bts@execbs.com
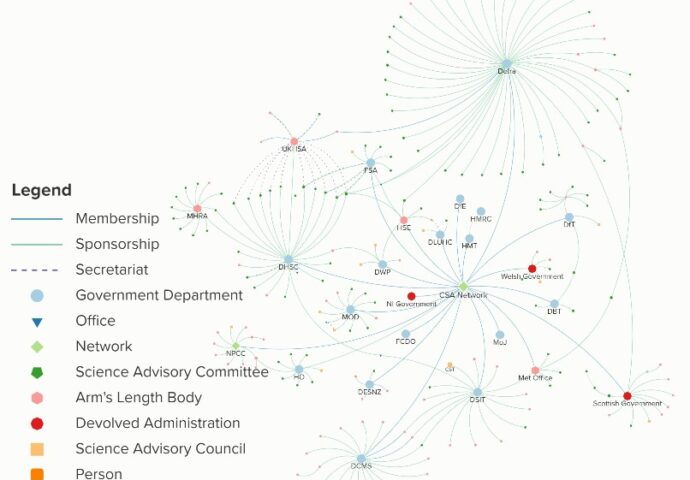
Government department committee map
This graphic shows the Government Office for Science’s systems map of the science advice committees, councils and arm’s length bodies associated with government departments. Use the button to interact with the map on the GO-Science website.
Visit the interactive mapThis committee assesses and provides advice to the FSA or other Government Departments on applications for the authorisation of new food additives or ingredients (including colours, preservatives, flavourings), enzymes and processing aids and on other topics relevant to the assessment of such applications as requested. The group coordinates with and considers the opinions of other relevant bodies concerned with the assessment of food additives and provides advice on general principals or new scientific discoveries in connection with the authorisation of food additives.
The ACAF advises on the safety and use of animal feeds and feeding practices, with particular emphasis on protecting human health, and with reference to new technical developments.
The ACDP has been established to provide independent expert advice in this area to the FSA and other departments.
https://www.gov.uk/government/groups/advisory-committee-on-dangerous-pathogens
This advisory committee is a non-statutory, independent body of scientific experts that advises the FSA on any matters relating to novel foods (including genetically modified foods) and novel processes (including food irradiation).
The group assesses and provides advice to the FSA or other government departments on applications for the authorisation of feed additives and substances used in animal feed for both animals and human consumers of animal derived products and on other topics relevant to the assessment of such applications as requested. The group coordinates with and considers the opinions of other relevant bodies concerned with the assessment of feed additives and provides advice on general principals or new scientific discoveries in connection with the authorisation of feed additives and substances.
The ASC is responsible for providing impartial, balanced and objective advice to the Secretary of State, to animal welfare bodies and within the European Union on issues relating to the Animals (Scientific Procedures) Act 1986 as amended. The Animals in Science Committee is an advisory non-departmental public body that provides independent advice to the Home Office.
https://www.gov.uk/government/organisations/animals-in-science-committee
This committee provides independent scientific advice on pesticide residues in the UK’s food. The Committee advises Ministers and our Chief Executives and the Chemicals Regulation Directorate (CRD) on the planning of surveillance programmes for pesticide residues in the UK food supply and the evaluation of the results, and procedures for sampling, sample processing and new methods of analysis.
https://www.gov.uk/government/groups/expert-committee-on-pesticide-residues-in-food-prif
The CHM advises ministers on the safety, efficacy and quality of medicinal products.CHM is an advisory non-departmental public body, sponsored by the Department of Health and Social Care.
https://www.gov.uk/government/organisations/commission-on-human-medicines
This committee assesses and gives advice on carcinogenic risk to humans.
The COM assesses and advises on mutagenic risks to humans.
This committee advises the government on all matters concerning the health effects of air pollutants and provides independent advice to government departments and agencies on how air pollution impacts on health.
https://www.gov.uk/government/groups/committee-on-the-medical-effects-of-air-pollutants-comeap
This is an independent scientific committee that provides advice to the FSA, the Department of Health and other Government Departments and Agencies on matters concerning the toxicity of chemicals.
HSAC provides expert advice on how to protect the environment, and human health via the environment, from potentially hazardous substances and articles, including nanomaterials.
https://www.gov.uk/government/groups/hazardous-substances-advisory-committee
HMAC advises on the safety and quality of herbal medicines when there’s an application for registration, marketing authorisation or product licence.
https://www.gov.uk/government/groups/herbal-medicines-advisory-committee
This group assesses and provides advice to the FSA on applications for the authorisation of new food contact materials including the materials, their chemical components, active and intelligent packaging and related recycling processes and on other topics relevant to the assessment of such applications as requested. The group coordinates with and considers the opinions of other relevant bodies concerned with the assessment of food contact materials and provides advice on general principles or new scientific discoveries in connection with food contact materials risks.
SQAG is a new group which will provide the HSE Chair, Board, Chief Executive and HSE Chief Scientific Adviser with independent and objective assurance on the relevance and quality of the science, engineering and evidence produced across HSE which is commissioned and delivered in support of HSE’s strategy and plans. This Group will undertake in-depth reviews of research and analysis conducted by HSE to provide an assurance function; it will not consider HSE policy or regulatory activities, and it will have no decision-making powers.
This advisory committee assesses and advises on chemical and biological risks to humans. SAG-CS is a temporary scientific advisory group, commissioned by the Office for Product Safety and Standards (OPSS). The mission of the SAG-CS is to provide OPSS with scientific advice and risk assessment in the areas of public health and consumer safety.
HSE acts as the Agency for UK REACH. Independent experts advise on the safety of chemicals and support the Agency’s scientific opinions.
https://www.hse.gov.uk/reach/reach-independent-scientific-expert-pool.htm
The VCP was originally established under the Medicines Act of 1968. Its remit derives from Article 28 of the Veterinary Medicines Regulation (VMR) 2013,The committee provides the Secretary of State with scientific advice on any aspect of veterinary medicinal products and specified feed additives.
https://www.gov.uk/government/organisations/veterinary-products-committee/about
Government Agencies may have either a Science Council or Advisory Board which are made up of independent members who provide high level, expert strategic insight, challenge and advice to the Chief Science Advisers (CSA) and the Executive Board . The purpose of these independent groups is to help ensure that the relevant Government Department identifies, sources, integrates and uses the best scientific evidence and expertise from all relevant disciplines to inform and evaluate the work.
Experts apply to join a register of pre-approved specialists from whom the relevant Government Department can call upon the individual to commission short pieces of ad hoc scientific work and/or invite them to join a committee, working group or advisory group to consider a specific item on a meeting Agenda to provide specific expertise to support the group considering the item with the ultimate aim in protecting the public.

